
ANNUAL REPORT 2019
of the state-owned enterprise
«State Expert Center of the Ministry of Health of Ukraine»
Our mission
To ensure the proper quality, efficiency and safety of medicines for Ukrainians and to create conditions for the sound pharmacotherapy in the healthcare sector.

Tetiana Dumenko
Director of the MoH State Expert Center
The year 2019 was rich in both challenges and successes. The State Expert Center was moving towards the fulfillment of its key tasks: to provide Ukrainians with high-quality, effective and safe medicines and to improve the attractiveness of our country to drug manufacturers through improved procedures.
It did not all go smoothly. Through fabricated criminal cases, some law enforcement units were pressuring the Center’s team. This can be regarded as an attack on transparency and openness in the work of the SEC.
Despite all the obstacles, 2019 brought several innovations.
We launched a new format of work – the Service Center and implemented a much more convenient approach to work with applicants. Over the past year, the Service Center became the face of our enterprise and welcomed about 50,000 visitors.
We developed a system called “Visualization” for clinical trial applicants so that they could track all stages of the examination of their applications and materials online.
We joined the European Health Technology Assessment Network. This allows us to adapt and use up-to-date international instruments to make informed decisions when selecting medicines for public procurement and regulatory listings.
In 2019, we once again proved that a state-owned enterprise can be successful. This translates into UAH 126 million paid in taxes and payments to the budget of Ukraine – 20% higher than planned.
For 2020, we set ambitious but achievable goals. This includes the introduction of electronic forms of communication: the applicant's account in the pharmacovigilance system, the electronic registration dossier and the full-scale electronic document flow.
None of the achievements of 2019 would have happened without the support of our partners and friends: Ministry of Health of Ukraine, State Service of Ukraine on Medicines and Drugs Control, Public Health Center, National Health Service of Ukraine, professional associations, international organizations and partners, and NGOs.
And finally, the achievement of 2019 is the result of the work of one team – the staff of the State Expert Center. Their professionalism, optimism and dedication to the common cause overcame the pressure and ensured that the tasks were fulfilled for the benefit of Ukrainian patients.
Key achievements of the State Expert Center in 2019

Opening of the Service Center
This is a new format of collaboration with applicants on the registration and re-registration of medicines, that made procedures fast, transparent and understandable for manufacturers

A breakthrough in the number of clinical trials in Ukraine
This happened thanks to the fruitful work of SEC experts on improving procedures for manufacturers

Membership in the European Health Technology Assessment Network
This will allow the use of up-to-date international decision-making tools in the selection of drugs for public procurement and regulatory lists.
SEC is a team of 614 experts and other people, seeking to make change here and now for the sake of patients, manufacturers and the state

for PATIENTS
to provide quality, safe and effective medication to every patient

for MANUFACTURERS
to create equal and transparent rules on the pharmaceutical market

for THE STATE
to build the investment attractiveness of Ukraine and pave the way to innovative medicines
FOR THE PATIENT
SEC works to provide patients with access to quality, safe and effective medicines

We have registered and re-registered medicines

We have provided access to innovative drugs through clinical trials

We have daily updated instructions in the State Register for medical use of medicines
Registration and re-registration of medicines



893 safe, quality and effective medicines received expert conclusions for registration in Ukraine. Due to the work of the SEC, today there are about 14,000 medicines on the Ukrainian market (10% more medicines registered compared to 2018).



27 854 messages of adverse reactions to medicines were collected online in 2019. In total, 226 077 such messages had been collected by SEC since 1996.
We timely remove drugs with dangerous side effects from circulation: when dangerous side effects occur, the cancellation of the drug registration is initiated.
Access to innovative medicines through clinical trials
As a result of 2019, 489 clinical trials are being conducted in Ukraine today. This opens the possibility to participate for 20 thousand patients who are thus able to be treated with innovative medicines for free, be under constant medical care and undergo free examinations.




Online access to information about medication and treatment
SEC experts regularly update information on registered medicines in the State Register. Since 2019, the patient can electronically report on adverse reactions or lack of effectiveness of drugs to the Center.
For the third year the SEC administers the Clinical Trials Registry in Ukraine. Thanks to its simple and accessible structure, patients can learn about opportunities to engage in research and receive innovative treatments for free under the supervision of medical researchers.
Since 2017, the SEC administers the Unified Register of Patients in Need of Insulin Therapy. This information system is simultaneously available for endocrinologists, representatives of pharmacies and regional Health Departments.
FOR THE MANUFACTURER
SEC aims to create equal opportunities for manufacturers to enter the pharmaceutical market through convenient and transparent online tools

We have registered medicines by simplified and expedited procedures

We have digitalized the procedures

We have increased the transparency of clinical trial expertise

We opened the Service Center
Simplified and expedited procedures for registering drugs


130 medicines were registered under a simplified procedure. It is almost 1.5 times (by 46%) more than last year. This means that medicines approved by the world's most stringent regulatory agencies (FDA, MHRA, EMA, PMDA, Swissmed) are getting to patients in Ukraine in shorter time. This is especially valuable when there are no similar drugs on the Ukrainian market.
Digitalization: shifting processes online
We improved the “Visualization” system (this is a system of free online access for applicants for tracking the examination process of their documents). We have put in place an electronic application form for changes to the Medicines Registration Certificate. These are the majority of applications the SEC receives. This has significantly reduced the time spent by experts for entering applications to the electronic database. The introduction of this form for drug re-registration is underway.
We upgraded Automated Pharmacovigilance Information System. Applicants can now create an electronic "Applicant's Account" in the System, download E2BR2 and E2BR3 xml files independently, and at any time receive information about adverse events after immunization, adverse reactions to medicines, vaccines, tuberculin they present to market of Ukraine.
Introducing transparent examination procedures for clinical trials
Currently, there are 489 clinical trials in Ukraine that can involve up to 20,000 patients in total.
To make this possible, SEC experts:
-
reviewed 304 applications for clinical trials
-
made 1743 major amendments to clinical trial protocols
-
provided 273 findings from examination and agreed to the approval of 249 clinical trial materials.
Opening of the SEC Service Center
At the beginning of March 2019, we introduced a new format for work with applicants, the Service Center.
This is a standard practice for European countries, but a new one for Ukraine, when a state expert institution switches to a service approach in work with medicines manufacturers.
-
On average, the Service Center receives 250 sets of documents daily, all requiring a checklist, as well as a number of separate documents. There are over 50,000 visits a year.
-
We reduced the number of overdue examinations of documents by 14-62% on different types of applications (registration, re-registration, changes) and by more than 90% on separate areas (security examination).




Impact of the work of the SEC Service Center:
-
We adjusted and systematized application algorithms. Applicants are now submitting their materials for examination when ready. They do not have to wait several weeks for an available appointment date by an expert.
-
For the SEC staff, the time they can allocate to the expertise has increased significantly.
-
We eliminated corruption risks.


FOR THE STATE
SEC is aiming at providing expertise of medicines, increasing investment attractiveness through equal competitive conditions for all market participants, self-sufficiency and contributing to the state budget with taxes and fees

We created conditions for investment in the Ukrainian economy

We contributed to the state budget

We introduced science-based approaches to decision-making practices

We created the conditions for the release of new innovative medicines
Ensuring implementation of international norms in Ukrainian legislation
The SEC is actively working to improve the image of the national clinical trial industry and increase its investment attractiveness.
In order to improve the examination procedure for the materials of clinical trials of medicines, to optimize its terms and to continue harmonization of legal acts of Ukraine with the legislation of the European Union, the Center submitted to the Ministry of Health the draft amendments and additions to the Procedure for conducting clinical trials of medicines (order 690). SEC experts developed these amendments based on the results of joint work of representatives of domestic and foreign manufacturers, the European Business Association and the researchers.
In 2019, the number of approved international clinical trials of medicines in Ukraine has crossed the threshold of 200 and reached 210 clinical trials. It happened for the first time since 2012 – since the entry into force of amendments to Article 3212 of the Criminal Code of Ukraine. The SEC argues that the article needs to be cancelled as its rules do not comply with international regulatory practice.
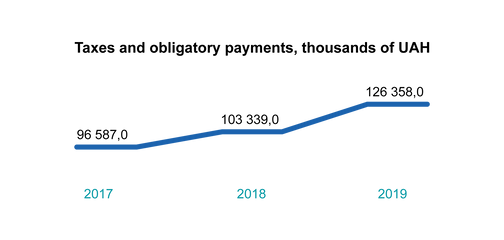
Contributing to the State Budget
We prove by our own financial results that a state-owned enterprise can be successful. We paid UAH 126.4 million to the state budget of Ukraine in 2019.
Introducing science-based approaches to decision-making
The SEC is constantly involved in the selection of drugs for the public procurement. Specialists of the Center in 2019:
-
Examined the impact on the budget of the inclusion of a particular drug in the National List of Essential Medicines. For this purpose, we have developed a special tool – the budgetary impact analysis calculator.
-
Worked to define criteria for prioritizing the drugs proposed for evaluation.
-
Developed recommendations on the level of impact on the budget.
Created the Health Technology Assessment Department and joined the European Health Technology Assessment Network to adapt to local conditions and use most up-to-date international decision-making tools and methods: pharmacoeconomic analysis and modeling, budget impact scale, multicriteria decision making analysis.
EXPANSION OF THE EXPERT POTENTIAL FOR THE CENTER AND THE INDUSTRY
Continuous professional development of the SEC specialists
Studied the experience of foreign countries on the materials examination and medicines registration, contributed to the organization and holding of international symposia, conferences, seminars.
The SEC's bioequivalence management specialist is involved as an expert in WHO assessments.
Experience exchange with specialists in Ukraine and from abroad
Pharmacovigilance conference was held with the participation of representatives of regulatory authorities of Ukraine and 6 CIS countries (Belarus, Moldova, Azerbaijan, Armenia, Uzbekistan), representatives of WHO European Regional Office, scientists and representatives of pharmacovigilance of Great Britain, Switzerland, experts from the Netherlands, Germany and the USA.
We actively worked with applicants, drug developers, and bioequivalence researchers: regularly held training with SEC experts. Particularly, to clarify the new guidelines on bioequivalence studies.
SEC experts trained specialists in Azerbaijan on approximation of national legislation to the EU legislation.
Created the website of “Pharmaceutical Journal”: pharmj.org.ua, which contains scanned PDF versions of articles for the years 1959-2010, as well as all files for 2019.
Audit of the work of the Center
We underwent a supervisory audit to confirm the compliance of the Center's quality management system with the requirements of the international standard ISO 9001: 2015.
We underwent an independent financial audit.
We constantly work to assess, identify and eliminate corruption risks in the work of the Center.
The experience the Center has gained and the potential accumulated allow to face present-day challeges, among others preventing the spread of COVID-19.
All this is done to ensure that the patient has access to safe, quality and effective medicines.
We share our achievements with our constant partners: the Ministry of Health of Ukraine, State Service of Ukraine on Medicines and Drugs Control, Public Health Center, National Health Service of Ukraine, professional associations, international partners.
Thank you for your assistance in completing our mission.
We believe that in 2020 we will jointly do everything possible for the sake of the health of Ukrainian patients!
State Expert Center of the Ministry of Health of Ukraine works to provide Ukrainians with quality, effective and safe medicines.

